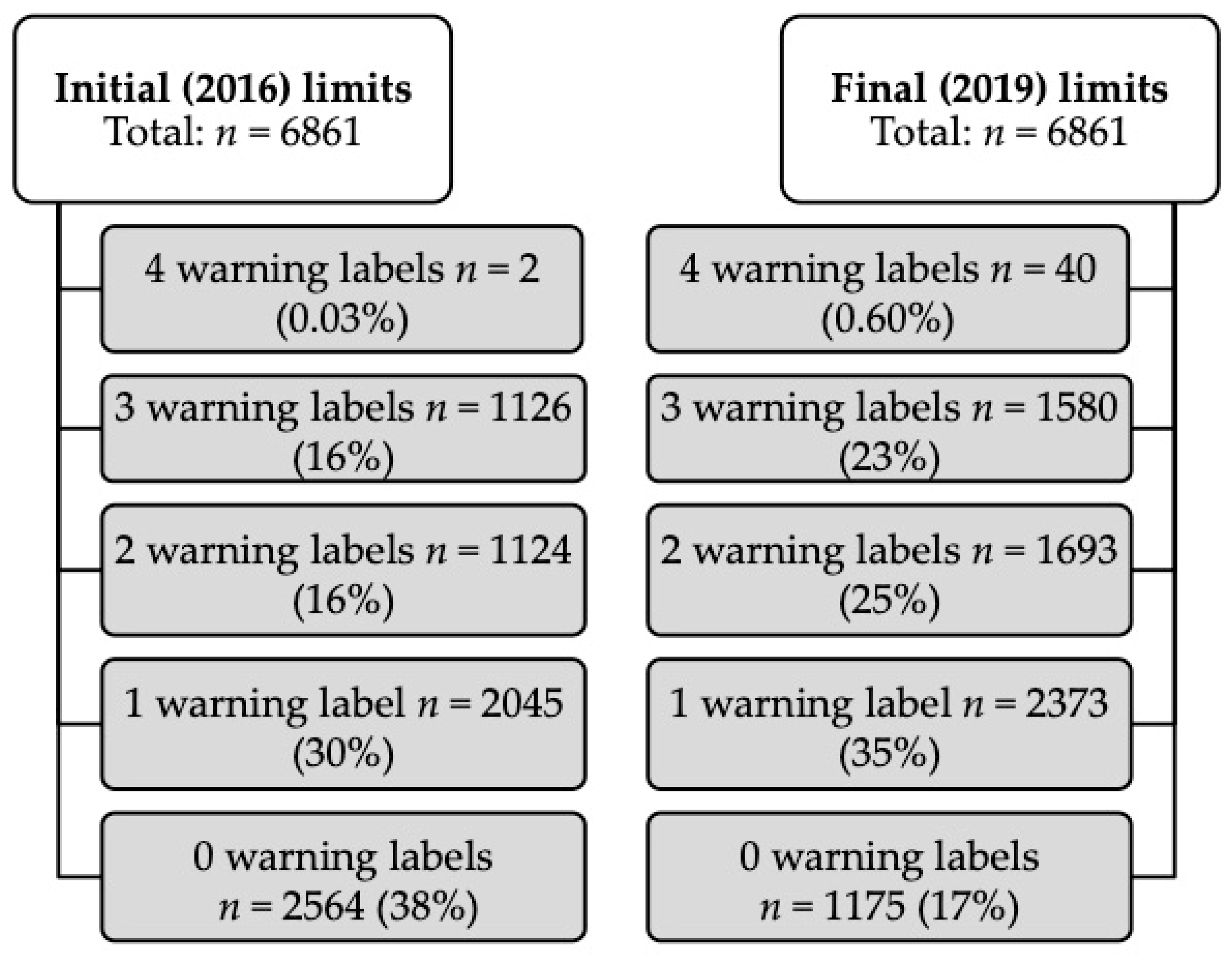
44 warning labels on vaccines
Black Box Drugs | FDA Warning Information - ConsumerSafety.org Dec 08, 2017 · MedWatch should not be used for reporting issues with vaccines, investigational drugs (still under study), or dietary supplements. Mail It In: The FDA also provides a PDF of the report form, which you can print, fill out, and send back to them. Again, this form is only for approved medications, not supplements or vaccines. Vaccines Licensed for Use in the United States | FDA Jul 05, 2022 · The product name and trade name of vaccines licensed for use in the United States.
Myocarditis and Pericarditis After mRNA COVID-19 Vaccination Sep 27, 2022 · The White House announced that vaccines will be required for international travelers coming into the United States, with an effective date of November 8, 2021. For purposes of entry into the United States, vaccines accepted will include FDA approved or authorized and WHO Emergency Use Listing vaccines. More information is available here.
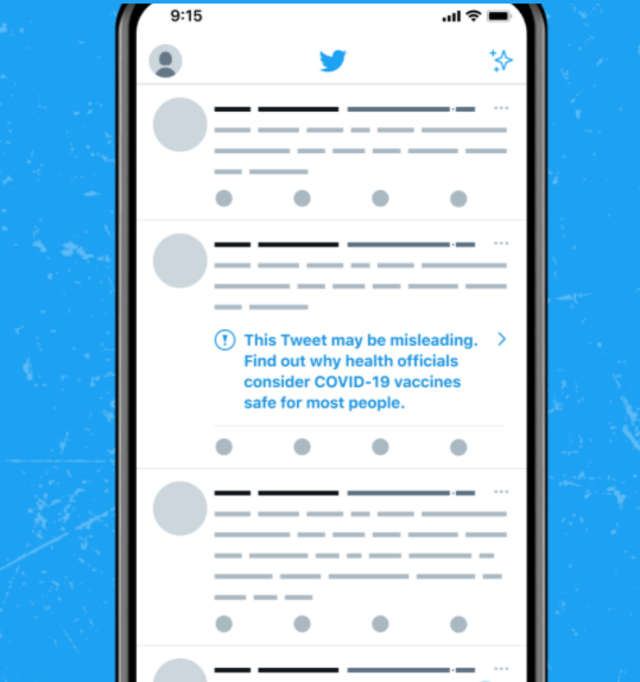
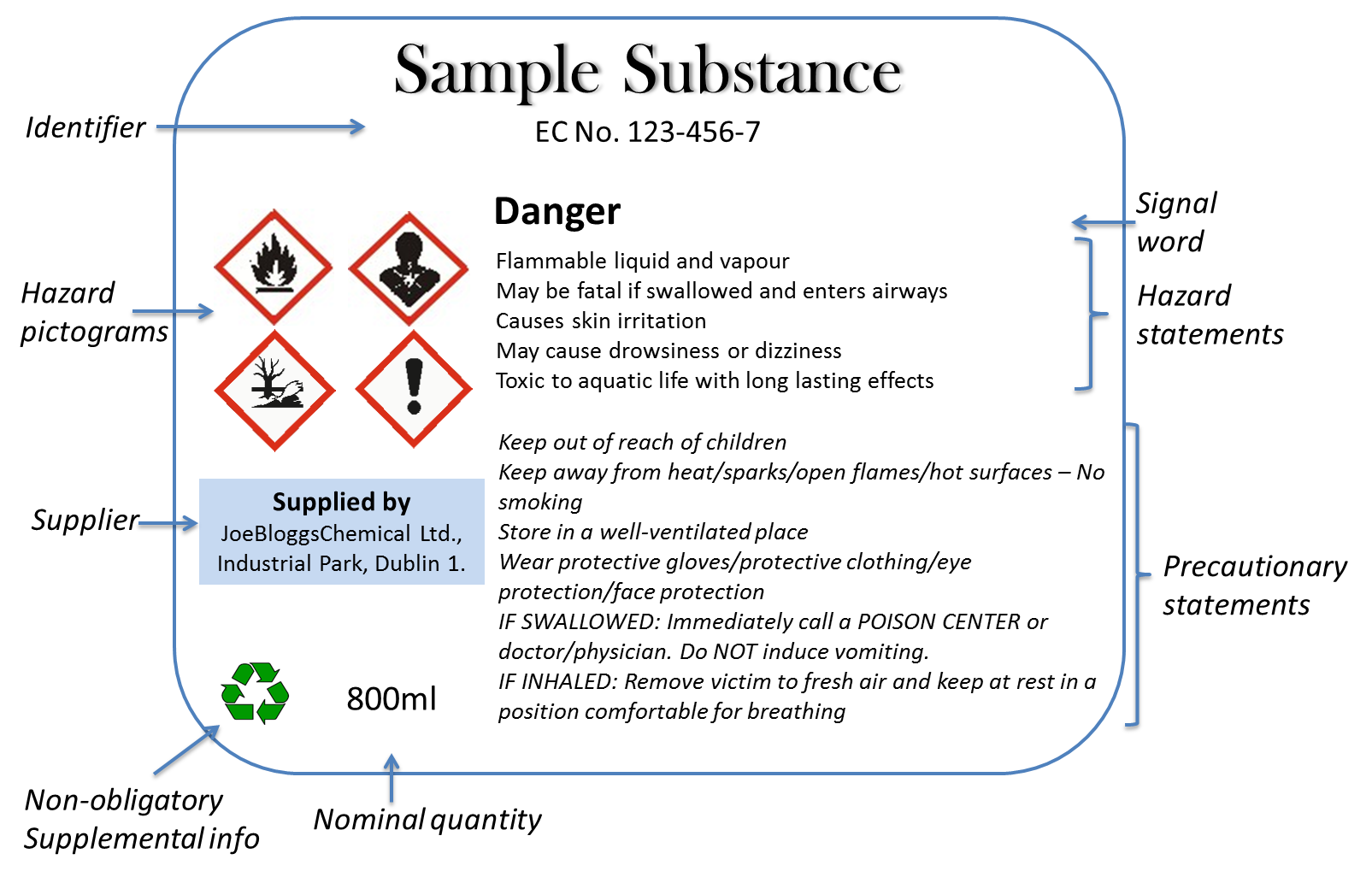
Warning labels on vaccines
FDA requiring Boxed Warning updated to improve safe use of ... Oct 02, 2020 · FDA is requiring the Boxed Warning be updated for all benzodiazepine medicines to include warnings about the risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions. Do ... Labeling and Warning Statements for Tobacco Products | FDA 1. The United States District Court for the District of Columbia recently issued an order vacating the health warning requirements for cigars and pipe tobacco set forth in 21 CFR §§ 1143.3 and ... FDA updates warnings for oral and injectable fluoroquinolone [07-26-2016] The U.S. Food and Drug Administration (FDA) approved changes to the labels of fluoroquinolone antibacterial drugs for systemic use (i.e., taken by mouth or by injection).
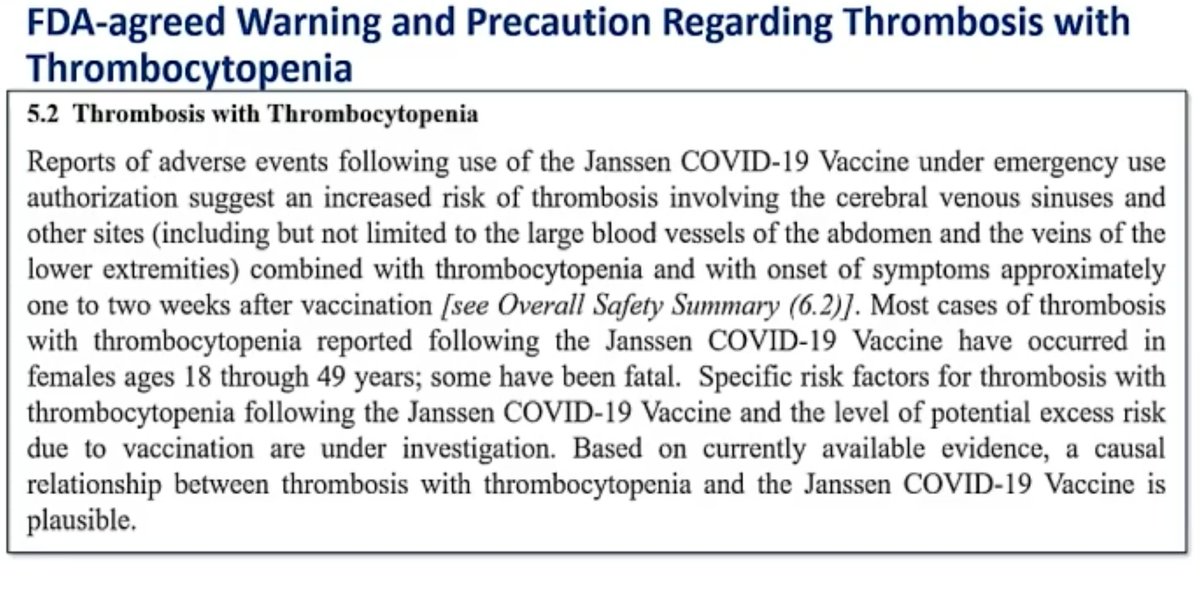
Warning labels on vaccines. FDA Requires a Warning about Guillain-Barré Syndrome (GBS) be ... FDA has required a new warning about GBS in the Prescribing Information for Shingrix as a result of new safety data from a postmarketing observational study. In the study, an increased risk of GBS ... FDA updates warnings for oral and injectable fluoroquinolone [07-26-2016] The U.S. Food and Drug Administration (FDA) approved changes to the labels of fluoroquinolone antibacterial drugs for systemic use (i.e., taken by mouth or by injection). Labeling and Warning Statements for Tobacco Products | FDA 1. The United States District Court for the District of Columbia recently issued an order vacating the health warning requirements for cigars and pipe tobacco set forth in 21 CFR §§ 1143.3 and ... FDA requiring Boxed Warning updated to improve safe use of ... Oct 02, 2020 · FDA is requiring the Boxed Warning be updated for all benzodiazepine medicines to include warnings about the risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions. Do ...




















/GettyImages-1306191744-3ff77276f77749c88148e3156b6f55d6.jpg)



Post a Comment for "44 warning labels on vaccines"